Rethink the monies spent on cancer screening tests
Posted: November 24, 2023 Filed under: behavior, cancer, Evolutionary perspective, healing, health, Nutrition/diet, self-healing | Tags: breast canceer, Cancer screening, environmental toxins, Life style, mammography, organic foods Leave a commentErik Peper, PhD and Richard Harvey, PhD
Cancer screening tests are based upon the rational that early detection of fatal cancers enables earlier and more effective treatments (Kowalski, 2021), however, there is some controversy. Early screening may increase the risk of over diagnosis, treating false positives (people who did not have the cancer but the test indicates they have cancer) and potentially fatal treatment of cancers that would never progress to increase morbidity or mortality (Kowalski, 2021).
Today about $40 billion spent on colon cancer screening, $15 billion spent on breast cancer screening, and $4 billion spent on prostate cancer screening annually (CSPH, 2021). A question is raised whether the billions and billions of dollars spent on screening asymptomatic participants would be more wisely spent on promoting and supporting life style changes that reduce cancer risks and actually extend life span? That cancer screening is expensive does not mean no one should be screened. Instead, the argument is that the majority of healthcare dollars could be spent on health promotion practices and reserving screening for those people who are at highest risk for developing cancers.
What is the evidence that screening prolongs life?
Cancer screening tests appear correlated with preventing deaths since deaths due to cancers in the USA have decreased by about 28% from 1999 to 2020 (CDC, 2023a). Although cancer causes many of the deaths in the USA, overall life expectancy has increased by less than 1% from 1999 to 2020. If cancer screening were more effective, the life expectancy should have increased more because cancer is the second leading cause of death (CDC, 2023b). Consider also that deaths due to cancers may be coincident and or comorbid with other circumstances. For example, during the last four years, overall life expectancy in the USA has precipitously declined in part due to other causes of death such as the COVID pandemic and opioid overdose epidemic (Lewis, 2022). Decline in life expectancy in the USA has many contributing factors, including the ‘harms’ associated with cancer screening procedures. For example, perforations during colon cancer screening can lead to internal bleeding, or complications related to surgeries, radiotherapies or chemotherapies. Bretthauer et al., (2023) commented: “A cancer screening test may reduce cancer-specific mortality but fail to increase longevity if the harms for some individuals outweigh the benefits for others or if cancer-specific deaths are replaced by deaths from competing cause” (p. 1197).
Bretthauer et al. (2023) conducted a systematic review and meta-analysis of 18 long-term randomized clinical trials involving 2.1 million Individuals with more than nine years of follow-up reporting on all-cause mortality. They reported that“…this meta-analysis suggest that current evidence does not substantiate the claim that common cancer screening tests save lives by extending lifetime, except possibly for colorectal cancer screening with sigmoidoscopy.”
Following is a summary of Bretthauer et al. (2023) findings:
- The only cancer screening with a significant lifetime gain (approximately 3 months) was sigmoidoscopy.
- There was no significant difference between harms of screening and benefits of screening for:
- mammography
- prostate cancer screening
- FOBT (fecal occult blood test) screening every year or every other year
- lung cancer screening Pap test cytology for cervical cancer screening, no randomized clinical trials with cancer-specific or all-cause mortality end points and long term follow-up were identified.
Potential for loss or harm (e.g., iatrogenic and nosocomial) versus potential for benefit and extended life
More than 35 years ago a significant decrease in breast cancer mortality was observed after mammography was implemented. The correlation suggested a causal relationship that screening reduced mortality (Fracheboud, 2004). This correlation made logical sense since the breast cancer screening test identified cancers early which could then be treated and thereby would result in a decrease in mortality.
How much money is spent on screening that may correlate with unintended harms?
The annual total expenditure for cancer screening is estimated to be between $40-$50 billion annually (CSPH, 2021). Below are some of the estimated expenditures for common tests other than colorectal cancer screening, which arguably is costly; however, has potential benefits that outweigh potential harms.
- $10.37-13.94 billion for mammography to screen 50% of eligible women (Badal et al, 2023).
- $2-4 billion for prostate cancer (Ma et al., 2014)
- $1-2 billion for lung cancer (Taylor et al., 2022)
What is the correlation between initiation of mammography and decrease in breast cancer mortality?
The conclusion that mammography reduced breast cancer mortality was based upon studies without control groups; however, this relationship could be causal or synchronistic. The ambiguity of correlation or causation was resolved with the use of natural experimental control groups. Some European countries began screening 10 years earlier than other countries. Using statistical techniques such as propensity score matching when comparing the data from countries that initiated mammography screening early (Netherlands, Sweden and Northern Ireland) to countries that started screening 10 year later (Belgium, Norway and Republic of Ireland), the effectiveness of screening could be compared.
The comparisons showed no difference in the decrease of breast cancer mortality in countries that initiated breast cancer screening early or late. For example, there was no difference in the decrease of breast cancer mortality rates of women who lived in the Netherlands that started screening early versus those who lived in Belgium that began screening 10 years later, as is shown Figure 1 (Autier et al, 2011).
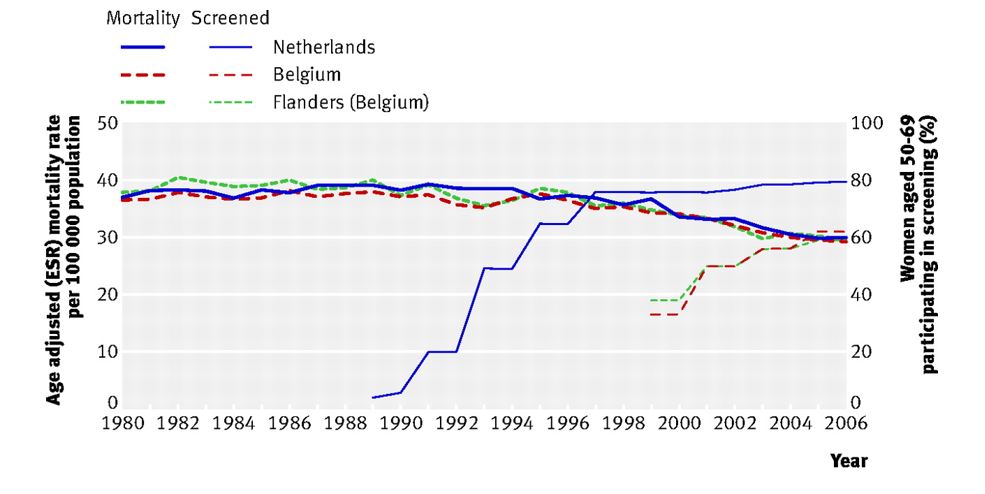
Figure 1. No difference in age adjust breast cancer mortality between the two adjacent countries even though breast cancer screening began ten years earlier in the Netherlands than in Belgium (graph reproduced from Autier et al, 2011).
The observations are similar when comparing neighboring countries: Sweden (early screening) to Norway (late screening) as well as Northern Ireland, UK (early screening) compared to the Republic of Ireland (late screening). The systematic comparisons showed that screening did not account for the decrease in breast cancer mortality. To what extent could the decrease in mortality be related to other factors such as better prenatal and early childhood diet and life style, improved nutrition, reduction in environmental pollutants, and other unidentified life style and environmental factors which improve immune competence?
A simplistic model to reduce the risk of cancers is described in the following equation (Gorter & Peper, 2011).

Cancer risk can be reduced, arguably by influencing risk factors that contribute to cancers as well as increasing factors to enhance immune competence. In the simple model above, ‘Cancer burden’ refers to the set of exposures that increase the odds of cancer formations. Categories include exposures to oncoviruses, environmental exposures (e.g., ionizing radiation, carcinogenic chemicals) as well as genetic (e.g., chromosomal aberrations, replication errors) and epigenetic factors (e.g., lifestyle categories related to eating, exercising, sleeping, and relaxing). In the model above, ‘Immune competence’ refers to a set of categories of immune functioning related to DNA repair, orderly cell death (i.e., processes of apoptosis), expected autophagy, as well as ‘metabolic rewiring,’ also called cellular energetics, that would allow the body to be able to reduce manage cancers from progressing (Fouad & Aanei, 2017) .
How do we examine the cancer burden/immune competence relationship?
Schmutzler et al., (2022) have suggested personalized and precision-medicine risk-adjusted cancer screening incorporating “… high-throughput “multi-omics” technologies comprising, among others, genomics, transcriptomics, and proteomics, which have led to the discovery of new molecular risk factors that seem to interact with each other and with non-genetic risk factors in a multiplicative manner.” The argument is that ‘profit-centered’ medicine could incorporate ‘multi-omics’ into risk-adjusted cancer screening as a way to reduce potential loss or harm due to other cancer screening procedures. Rather than simply screening for cancers using currently invasive or toxic procedures which may do more harm than good, consider more nuanced screening tests aimed at the so-called ‘hallmarks of cancer?’ For example, Hanahan (2022) suggests some technical targets for the multi-omics technologies. The following are some of the precision screening tests possible topersonalized medicine of 14 factors or processes related to:
- cells evading growth suppression
- non-mutational epigenetic reprogramming
- avoiding immune destruction
- enabling replicative immortality
- tumor-promoting inflammation
- polymorphic microbiomes
- activating invasion and metastasis
- inducing or accessing vasculature formation/angiogenesis
- cellular senescence
- genome instability and mutation
- resisting cell death
- deregulating cellular metabolism
- unlocking phenotypic plasticity
- sustaining proliferative signaling
Of the listed categories above, ‘phenotypic plasticity’ (cf. Feinberg, 2007; Gupta et al., 2019) suggests that lifestyle behaviors and environmental exposures play a role in cancer progression and regression.
Lifestyle and environmental factors can contribute to the development of cancers.
The 2008-2009 report from the President’s Cancer Panel appraised the National Cancer Program in accordance with the National Cancer Act of 1971 stated (Reuben, 2010):
“We have grossly underestimated the link between environmental toxins, plastics, chemicals, and cancer risk. It is estimated that 70 percent of all cancers were related to diet and environment “
Multiple research studies have shown that a healthy life style pattern is associated with decreased cancer risks and increased longevity. Lifestyle factors that have been documented to increase cancer risks in the United Kingdom (UK) as shown in figure 2.
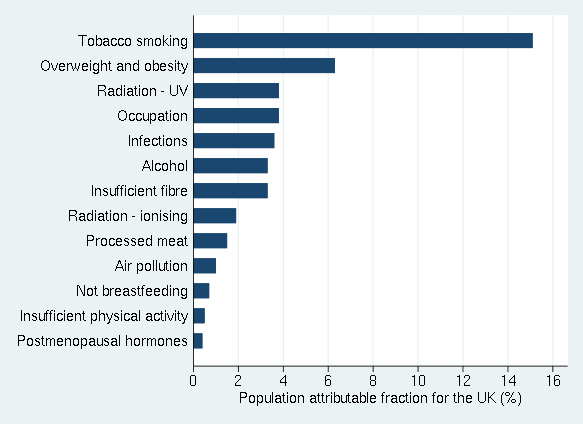
Figure 2. Percentages of cancer cases in the UK attributable to different exposures. Adapted from Brown et al., 2018 and reproduced by permission from Key et al., 2020.
Similar findings have been reported by Song et al. (2016) from the long term follow-up of 126901 adult health care professionals. People who never smoked, drank no alcohol or moderate alcohol (< 1 drink/d for women; < 2 drinks/d for men}, had a body-mass index (BMI) of at least 18.t but lower than 27.5, did weekly aerobic physical activity of at least 75 vigorous-intensity minutes or 150 150 moderate-intensity minutes compared to those who smoked, drank, had high BMI and did not exercise had nearly half the cancer death rate. Song et al (2016) concludes:
“…about 20% to 40% of carcinoma cases and about half of carcinoma deaths can be potentially prevented through lifestyle modification. Not surprisingly, these figures increased to 40% to 70% when assessed with regard to the population of US whites which has a much worse lifestyle pattern than other cohorts. Notably, approximately 80% to 90% of lung cancer deaths can be avoided if Americans adopted the lifestyle of the low-risk group, mainly by quitting smoking. For other cancers, a range of 10% to 70% of deaths can be prevented. These results provide strong support for the importance of environmental factors in cancer risk and reinforce the enormous potential of primary prevention for cancer control.”
Said another way, primary prevention should remain a priority for cancer control.
Given that many cancers are related to diet, environment and lifestyle, it is estimated that 50% of all cancers and cancer deaths could be prevented by modifying personal behavior. Thus, the monies spent on screening or even developing new treatments could better be spent on prevention along with implementing programs that promote a healthier environment, diet and personal behavior (AACR, 2011).
What can be done? Addressing systems not symptoms
From a ‘systems perspective,’ the first step is to reduce the cancer burden and carcinogenic agents that occur in our environment such environmental pollution (Turner et al., 2022). In many cases, governmental regulations that reduce cancer risk factors have been weakened, delayed, and contested for years through industry’s lobbying. It often takes more than 30 years after risk factors have been observed and documented before government regulations are successfully implemented, as exemplified in the battle over tobacco or, air pollution regulations related to particulates from burning fossil fuels (Stratton et al, 2001).
Sadly, we cannot depend upon governments or industries to implement regulations known to reduce cancer risks. More within our control is implementing lifestyle changes that enhance immune competence and promote health.
Implement a healthy life style that enhances immune competence and, supports health and well-being
Paraphrasing a trope of what some physicians may state: ‘Take two pills, and call me in the morning. Oh, and eat well, exercise, and get good rest.’ Broadly stated, the following are some controllable lifestyle behaviors that can decrease cancer risks and promotes health. Implementing environmental and lifestyle changes are very challenging because they are highly related to socio economic factors, cultural factors, industry push for profits over health, and self-care challenges since there are no immediate results experienced by behavior and lifestyle changes.
In many cases, the effects of harmful life-style and environment factors are only observed twenty or more years later (e.g., diabetes, lung cancer, cirrhosis of the liver). The individual does not experience immediate benefits of lifestyle changes thus it is more challenging to know that your healthy life style has an effect. The process is even more complex because in most cases it is not a single factor but the interaction of multiple factors (genetics, lifestyle, and environment). The complexity of causality so often conflicts with the simplistic research studies to identify only one isolated risk factor. Instead of waiting for the definitive governmental guidelines and regulations, adopt a ‘precautionary principle’ which means do not take an action when there is uncertainty about its potential harm (Goldstein, 2001). Do not wait for screening; instead, take charge of your health and implement as many of the following behaviors and strategies to enhance immune competence and thereby reduce cancer risks.
Eat organic foods, especially vegetable and fruits.
Many studies have suggested that eating organic foods and in particular more fruits and vegetable such as a Mediterranean diet is associated with increased health and longevity. Similarly, people who eat do not eat highly-processed or ultra-processed foods have better health status (Van Tulleken, 2023). For example, In the large prospective study of 68, 946 participants, adults who consumed the most organic fruits, vegetables, dairy products, meat and other foods had 25% fewer cancers when compared with adults who never ate organic food (Baudry et al., 2018; Rabin, 2018). Similarly, many studies have reported that those who adhere consistently to a Mediterranean diet have a significantly lower incidence of chronic diseases (such as cardiovascular diseases, diabetes, etc.) and cancers compared to those who do not adhere to a Mediterranean diet (Mentella et al., 2019).
Reduce environmental pollution exposure
Air pollution and the exposure to airborne carcinogens are a significant risk factor for cancers as illustrated by the increased cancer rates among smokers. In the USA, the reduction of smoking has significantly decreased the lung cancer deaths (US Department of Health and Human Services, 2014).
Increase movement and exercise
Many studies have documented that people who exercise regularly and are otherwise non–sedentary but are active their entire lives have the lowest risk for breast cancers and colon cancers. Women who exercise 3 hours a week or more have a 30-40% lower risk of developing breast cancer (NIH NCI, 2023). The NIH National Cancer Institute summary concludes that exercises also significantly benefited the following cancer survivors (NIH NCI, 2023):
- Breast cancer: In a 2019 systematic review and meta-analysis of observational studies, breast cancer survivors who were the most physically active had a 42% lower risk of death from any cause and a 40% lower risk of death from breast cancer than those who were the least physically active (Spei et al, 2019).
- Colorectal cancer: Evidence from multiple epidemiologic studies suggests that physical activity after a colorectal cancer diagnosis is associated with a 30% lower risk of death from colorectal cancer and a 38% lower risk of death from any cause (Patel et al., 2019).
- Prostate cancer: Limited evidence from a few epidemiologic studies suggests that physical activity after a prostate cancer diagnosis is associated with a 33% lower risk of death from prostate cancer and a 45% lower risk of death from any cause ((Patel et al., 2019).
- Implement stress management.
Chronic stress may reduce immune competence and increase the risk of cancers as well as hinders healing from cancer treatments (Dai et al., 2020). The results of numerous studies have shown that implementing stress management spractices uch as Cognitive-behavioral stress management (CBSM) improves mood and lowers distress during treatment and, is also associated with longer survival compared to control groups in the 8-15 year follow up (Stagl et al., 2015).
Respect your biological rhythm.
The International Agency for Research on Cancer (IARC) reports that, when the human circadian clock is disrupted, the likelihood of developing cancers, including lung cancers, intestinal cancers, and breast cancers, dramatically increases (Huang, et al., 2023). Go to bed at the same time and, have about 8 hours of sleep. As much as possible avoid night shifts at work along with frequent jet lag as that highly disrupts the circadian rhythm.
Increase social connections and be part of a social community
Absence of social support, feeling lonely and socially isolated tends reduces immune competence and increases cancer mortality risk while having more social support satisfaction is associated with lower mortality risks (Salazaor et al., 2023; Boen et al., 2018). Meta-analysis of 148 studies (308,849 participants) found that that on the average there is a 50% increased likelihood of survival for participants with stronger social relationships (Holt-Lunstad et al., 2010).
Live a life with meaning and purpose
Having meaning and purpose make each moment worth living and may contribute to improving immune function and possible cancer survival (LeShan, 1994; Rosenbaum & Rosenbaum, 2023).
Summary
The research suggests that cancer screening does not extend life span unless specifically performed for certain diagnostic purposes or, with individuals who are at high risk of developing cancers (e.g., have a genetic predisposition). Implementing self-care health behaviors that have been identified to promote health and increase lifespan. Implementing health behaviors is challenging since there is limited governmental and corporate support. Thus, take charge and implement a holistic self-care lifestyle that reduces cancer risk factors and supports health.
See also the following blogs:
References
AACR. (2011). AACR Cancer Progress Report 2011. American Association for Cancer Research. http://www.aacr.org/Uploads/DocumentRepository/2011CPR/2011_AACR_CPR_Text_web.pdf
American Cancer Society. (2021). History of ACS Recommendations for the Early Detection of Cancer in People Without Symptoms. Accessed November 11, 2023. https://www.cancer.org/health-care-professionals/american-cancer-society-prevention-early-detection-guidelines/overview/chronological-history-of-acs-recommendations.html
Autier, P., Boniol, M., Gavin, A,, & Vatten, L.J. (2011) Breast cancer mortality in neighbouring European countries with different levels of screening but similar access to treatment: trend analysis of WHO mortality database. BMJ. 343, d4411. https://doi.org/10.1136/bmj.d4411
Badal., K., Staib, J., Tice,J., Kim, M-O., Eklund, M., DaCosta Byfield, S., Catlett,K., Wilson,L., et al, (2023). Cost of breast cancer screening in the USA: Comparison of current practice, advocated guidelines, and a personalized risk-based approach. Journal of Clinical Oncology, 41: 16_suppl, 3 18917 :16_suppl, e18917. https://doi.org/10.1200/JCO.2023.41.16_suppl.e18917
Baudry, J., Assmann, K.E., Touvier, M., et al. (2018). Association of Frequency of Organic Food Consumption With Cancer Risk: Findings From the NutriNet-Santé Prospective Cohort Study. JAMA Intern Med, 178(12), 1597–1606. https://doi.org/10.1001/jamainternmed.2018.4357
Boen, C.E., Barrow, D..A, Bensen, J.T., Farnan, L., Gerstel, A., Hendrix, L.H., Yang, Y.C. (2018). Social Relationships, Inflammation, and Cancer Survival. Cancer. Epidemiol Biomarkers Prev, 27(5), 541-549. https://doi.org/10.1158/1055-9965.EPI-17-0836
Bretthauer M, Wieszczy P, Løberg M, et al. (2023). Estimated Lifetime Gained With Cancer Screening Tests: A Meta-Analysis of Randomized Clinical Trials. JAMA Intern Med. 183(11),1196–1203. https://doi.org/10.1001/jamainternmed.2023.3798Brown, K.F., Rumgay, H., Dunlop, C. et al. (2018). The fraction of cancer attributable to modifiable risk factors in England, Wales, Scotland, Northern Ireland, and the United Kingdom in 2015. Br J Cancer, 118, 1130–1141. https://doi.org/10.1038/s41416-018-0029-6
CDC. (2023a). U.S. Cancer Statistics Working Group. U.S. Cancer Statistics Data Visualizations Tool, based on 2022 submission data (1999-2020): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; released in November 2023. https://www.cdc.gov/cancer/dataviz
CDC. (2023b). Leading Causes of Death. National Center for health statistics, Centers for disease control and prevention. Accessed November 20, 2023. https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm
CSPH. (2021). Estimating annual expenditures for cancer screening in the United States. Center for Surgery and Public Health. Assessed November 14, 2023. https://csph.brighamandwomens.org/wp-content/uploads/2021/12/Estimating-Annual-Expenditures-for-Cancer-Screening-in-the-United-States.pdf
Dai, S., Mo, Y., Wang, Y., Xiang, B., Liao, Q., Zhou, M., Li, X., Li, Y., Xiong. W., Li, G., Guo, C., & Zeng, Z. (2020). Chronic Stress Promotes Cancer Development. Front Oncol. 10, 1492. https://doi.org/10.3389/fonc.2020.01492
Feinberg, A. P. (2007). Phenotypic plasticity and the epigenetics of human disease. Nature, 447(7143), 433-440. https://doi.org/10.1038/nature05919
Fouad, Y. A., & Aanei, C. (2017). Revisiting the hallmarks of cancer. American journal of cancer research, 7(5), 1016. https://pubmed.ncbi.nlm.nih.gov/28560055/
Fracheboud, J. et al. (2004). Decreased rates of advanced breast cancer due to mammography screening in The Netherlands, British Journal of Cancer (2004) 91, 861–867. https://doi,org/10.1038/sj.bjc.6602075
Goldstein, B.D. (2001). The precautionary principle also applies to public health actions. Am J Public Health, 91(9),1358-61. https://doi.org/10.2105/ajph.91.9.1358
Gorter, R. & Peper, E. (2011). Fighting Cancer-A None Toxic Approach to Treatment. Berkeley: North Atlantic/New York: Random House. https://www.amazon.com/Fighting-Cancer-Nontoxic-Approach-Treatment/dp/1583942483
Gupta, P. B., Pastushenko, I., Skibinski, A., Blanpain, C., & Kuperwasser, C. (2019). Phenotypic plasticity: driver of cancer initiation, progression, and therapy resistance. Cell Stem Cell, 24(1), 65-78. https://doi.org/10.1016/j.stem.2018.11.011
Hanahan, Douglas. (2022): Hallmarks of cancer: new dimensions. Cancer discovery, 12(1), 31-46. https://doi.org/10.1158/2159-8290.CD-21-1059
Holt-Lunstad, J., Smith, T.B., & Layton, J.B. (2010). Social Relationships and Mortality Risk: A Meta-analytic Review, PLoS Med 7(7), e1000316. https://doi.org/10.1371/journal.pmed.1000316
Huang, C., Zhang, C,, Cao, Y., Li, J., & Bi, F. (2023). Major roles of the circadian clock in cancer. Cancer Biol Med, 20(1):1–24. https://doi.org/10.20892/j.issn.2095-3941.2022.0474
Kalaf, J.M. (2014). Mammography: a history of success and scientific enthusiasm. Radiol Bras. 47(4):VII-VIII. https://doi.org/10.1590/0100-3984.2014.47.4e2
Key TJ, Bradbury KE, Perez-Cornago A, Sinha R, Tsilidis KK, Tsugane S. Diet, nutrition, and cancer risk: what do we know and what is the way forward? BMJ. 2020 Mar 5;368:m511. https://doi.org/10.1136/bmj.m511
Kowalski, A.E. (2021). Mammograms and mortality: How has the evidence evolved? J Econ Perspect, 35(2), 119-140. https://doi.org/10.1257/jep.35.2.119
LeShan, L. (1994). Cancer as a turning point. New York: Plume. https://www.amazon.com/Cancer-As-Turning-Point-Professionals/dp/0452271371
Lewis, T. (2022). The U.S. just lost 26 years’ worth of progress on life expectancy. Scientific American. October 17, 2022. Accessed November 11, 2023. https://www.scientificamerican.com/article/the-u-s-just-lost-26-years-worth-of-progress-on-life-expectancy/
Ma, X., Wang, R., Long, J.B., Ross, J.S., Soulos, P.R., Yu, J.B., Makarov, D.V., Gold, H.T. and Gross, C.P. (2014), The cost implications of prostate cancer screening in the Medicare population. Cancer, 120: 96-102. https://doi.org/10.1002/cncr.28373
Mentella, M.C., Scaldaferri, F., Ricci, C., Gasbarrini, A., & Miggiano, G.A.D. (2019). Cancer and Mediterranean Diet: A Review. Nutrients,11(9):2059. https://doi.org/10.3390/nu11092059
NIH NCI (2023). Physical Activity and Cancer. National Institutes of Health National Cancer Institute. Accessed November 18, 2023. https://www.cancer.gov/about-cancer/causes-prevention/risk/obesity/physical-activity-fact-sheet
Patel, A,V., Friedenreich, C.M., Moore, S.C, et al. (2019). American College of Sports Medicine Roundtable Report on physical activity, sedentary behavior, and cancer prevention and control. Medicine and Science in Sports and Exercise, 51(11), 2391-2402. https://doi.org/10.1249/MSS.0000000000002117
Rabin, R.C. (2018). Can eating organic food lower your cancer risk? The New York Times. Oct 23, 2018. Accessed November 17, 2023. https://www.nytimes.com/2018/10/23/well/eat/can-eating-organic-food-lower-your-cancer-risk.html
Reuben, S.H. (2010). Reducing environmental cancer risk – What We Can Do Now. The President’s Cancer Panel Report. Washington, D.C: U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES, National Institutes of Health, National Cancer Institute. https://deainfo.nci.nih.gov/advisory/pcp/annualReports/pcp08-09rpt/PCP_Report_08-09_508.pdf
Rosenbaum, E. H. & Rosenbaum, I.R. (2023) The Will to Live. Stanford Center for Integrative Medicine. Surviving Cancer. Accessed November 23, 2023. https://med.stanford.edu/survivingcancer/cancers-existential-questions/cancer-will-to-live.html
Salazar, S.M.D.C., Dino, M.J.S., & Macindo, J.R.B. (2023). Social connectedness and health-related quality of life among patients with cancer undergoing chemotherapy: a mixed method approach using structural equation modelling and photo-elicitation. J Clin Nurs. Published online March 9, 2023. https://doi.org/10.1111/jocn.16675
Schmutzler, R. K., Schmitz-Luhn, B., Borisch, B., Devilee, P., Eccles, D., Hall, P., … & Woopen, C. (2022). Risk-adjusted cancer screening and prevention (RiskAP): complementing screening for early disease detection by a learning screening based on risk factors. Breast Care, 17(2), 208-223. https://doi.org/10.1159/000517182
Song, M., & Giovannucci, E. (2016). Preventable incidence and mortality of carcinoma associated with lifestyle factors among white adults in the United States. JAMA Ooncology, 2(9), 1154-1161. https://doi.org/10.1001/jamaoncol.2016.0843
Spei, M.E., Samoli, E., Bravi, F., et al. (2019). Physical activity in breast cancer survivors: A systematic review and meta-analysis on overall and breast cancer survival. Breast, 44,144-152. https://doi.org/10.1016/j.breast.2019.02.001
Stagl, J.M., Lechner, S.C., Carver, C.S. et al. (2015). A randomized controlled trial of cognitive-behavioral stress management in breast cancer: survival and recurrence at 11-year follow-up. Breast Cancer Res Treat, 154, 319–328. https://doi.org/10.1007/s10549-015-3626-6
Stratton, K., Shetty, P., Wallace, R., et al., eds. (2001). Institute of Medicine (US) Committee to Assess the Science Base for Tobacco Harm Reduction. Washington (DC): National Academies Press (US). https://www.ncbi.nlm.nih.gov/books/NBK222369/
Tailor, T.D,, Bell, S., Fendrick, A.M., & Carlos, R.C. (2022) Total and Out-of-Pocket Costs of Procedures After Lung Cancer Screening in a National Commercially Insured Population: Estimating an Episode of Care. J Am Coll Radiol. 19(1 Pt A), 35-46. https://doi.org/10.1016/j.jacr.2021.09.015
Turner, M.C., Andersen, Z.J., Baccarelli, A., Diver, W.R., Gapstur, S.M., Pope, C.A 3rd, Prada, D., Samet, J., Thurston, G., & Cohen, A. (2020). Outdoor air pollution and cancer: An overview of the current evidence and public health recommendations. CA Cancer J Clin, 10.3322/caac.21632. https://doi.org/10.3322/caac.21632
US Department of Health and Human Services (2014). The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. Atlanta, GA: :
US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. https://aahb.org/Resources/Pictures/Meetings/2014-Charleston/PPT%20Presentations/Sunday%20Welcome/Abrams.AAHB.3.13.v1.o.pdf
Van Tulleken, C. (2023). Ultra-processed people. The science behind food that isn’t food. New Yoerk: W.W. Norton & Company. https://www.amazon.com/gp/product/1324036729/ref=ox_sc_act_title_1?smid=ATVPDKIKX0DER&psc=1