Reduce initial dose of the virus and optimize your immune system
Posted: April 4, 2020 Filed under: behavior, health, stress management, Uncategorized | Tags: coronavirus, COVID-19, immune system, public health, technology 9 CommentsErik Peper and Richard Harvey
Adapted from:Peper, E. & Harvey, R. (September 13, 2020). Reduce Initial Dose of the Virus and Optimize Your Immune System. Townsend Letters-The Examiner of Alternative Medicine, 44. https://www.townsendletter.com/article/reduce-initial-dose-of-the-virus-and-optimize-your-immune-system/
COVID-19 can sometimes overwhelm young and old immune systems and in some cases can result in ‘Severe Acute Respiratory Syndrome’ pneumonia and death (CDC, 2020). The risk is greater for older people, and people with serious heart conditions (e.g., heart failure, coronary artery disease, or cardiomyopathies), cancers, obesity, Type 2 diabetes, COPD, chronic kidney disease, hypertension, smoking, immune suppression or other health issues (CDC, 2020a) as well as young people who vape or smoke and those with immunological defects in type I and II interferon production (Gaiha, Cheng, & Halpern-Felsher, 2020; van der Made, 2020). As we age the immune system deteriorates (immunosenescence) that reduces the response of the adaptive immune system that needs to respond to the virus infection (Aw, Silva & Palmer, 2007; Osttan, Monti, Gueresi, et al., 2016). On the other hand, for young people and children the risk is very low and similar for Covid-19 as for seasonal influenza A and B in rates for hospitalization, admission to the intensive care unit, and mechanical ventilator ( Song et al, 2020).
Severity of disease may depend upon initial dose of the virus
In a brilliant article, How does the coronavirus behave inside a patient? We’ve counted the viral spread across peoples; now we need to count it within people, assistant professor of medicine at Columbia University and cancer physician Siddhartha Mukherjee points out that severity of the disease may be related to the initial dose of the virus. Namely, if you receive a very small dose (not too many virus particles), they will infect you; however, the body can activate its immune response to cope with the infection. The low dose exposure act similar to vaccination. If on the other hand you are exposed to a very high dose then your body is overwhelmed with the infection and is unable to respond effectively. Think of a forest fire. A small fire can easily be suppressed since there is enough time to upgrade the fire-fighting resources; however, during a fire-storm with multiple fires occurring at the same time, the fire-fighting resources are overwhelmed and there is not enough time to recruit outside fire-fighting resources.
As Mukherjee points out this dose exposure relationship with illness severity has a long history. For example, before vaccinations for childhood illnesses were available, a child who became infected at the playground usually experienced a mild form of the disease. However, the child’s siblings who were infected at home develop a much more severe form of the disease.
The child infected in the playground most likely received a relatively small dose of the virus over a short time period (viral concentration in the air is low). On the other hand, the siblings who were infected at home by their infected brother or sister received a high concentration of the virus over an extended period which initially overwhelmed their immune system. Higher virus concentration is more likely during the winter and in well insulated/sealed houses where the air is recirculated without going through HEPA or UV filters to sterilize the air. When there is no fresh air to decrease or remove the virus concentration, the risk of severity of illness may be higher (Heid, 2020).
The risk of becoming sick with COVID-19 can only occur if you are exposed to the coronavirus and the competency of your immune system. This can be expressed in the following equation. This equation suggests two strategies to reduce risk: reduce coronavirus load/exposure and strengthen the immune system.
This equation suggests two strategies to reduce risk: reduce coronavirus load/exposure and strengthen the immune system.
How to reduce the coronavirus load/dose of virus exposure
Assume that everyone is contagious even though they may appear healthy. Research suggests that people are already contagious before developing symptoms or are asymptomatic carriers who do not get sick and thereby unknowingly spread the virus (Furukawa, Brooks, Sobel, 2020). Dutch researchers have reported that, “The proportion of pre-symptomatic transmission was 48% for Singapore and 62% for Tianjin, China (Ganyani et al, 2020). Thus, the intervention to isolate people who have symptoms of COVID-19 (fever, dry cough, etc.) most likely will miss the asymptomatic carriers who may infect the community without awareness. Only if you have been tested, do you know if you been exposed or recovered from the virus. To reduce exposure to the virus, avoid the “Three C’s” — closed spaces with poor ventilation, crowded places and close contact—and do the following:
- Follow the public health guidelines:
-
- Social distance (physical distancing while continuing to offer social support)
- Wear a mask and gloves to reduce spreading the virus to others.
- Wash your hands with soap for at least 20 seconds.
- Avoid touching your face to prevent microorganisms and viruses to enter the body through mucosal surfaces of the nose mouth and eyes.
- Clean surfaces which could have been touched by other such as door bell, door knobs, packages.
- Avoid the person’s slipstream that may contain the droplets in the exhaled air. The purpose of social distancing is to have enough distance between you and another person so that the exhaled air of the other person would not reach you. The distance between people depends upon their activities and the direction of airflow.
In a simulation study, Professor Bert Blocken and his colleagues at KU Leuven and Eindhoven University of Technology reported that the plume of the exhaled air that potentially could contain the virus droplets could extend much more than 5 feet. It would depends upon the direction of the wind and whether the person is walking or jogging as show in Figure 1 (Blocken, 2020).

Figure 1. The plume of exhaled droplets that could contain the virus extends behind the person in their slipstream (photo from KU Leuven en TU Eindhoven).
The plume of exhaled droplets in the person’s slipstream may extend more than 15 feet while walking and more than 60 feet while jogging or bicycling. Thus. social distancing under these conditions is much more than 6 feet and it means avoiding their slipstream and staying much further away from the person.
- Increase fresh air to reduce virus concentration. The CDC recommends ventilation with 6 to 12 room air changes per hour for effective air disinfection (Nardell & Nathavitharana, 2020). By increasing the fresh outside air circulation, you dilute the virus concentration that may be shed by an infected asymptomatic or sick person (Qian & Zheng, 2018). Thus, if you are exposed to the virus, you may receive a lower dose and increase the probability that you experience a milder version of the disease. Almost all people who contract COVID-19 are exposed indoors to the virus. In the contact tracing study of 1245 confirmed cases in China, only a single outbreak of two people occurred in an outdoor environment (Qian et al, 2020). To increase fresh air (this assumes that outside air is not polluted), explore the following:
-
- Open the windows to allow cross ventilation through your house or work setting. One of the major reasons that the flu season spikes in the winter is that people congregate indoors to escape weather extremes. People keep their windows closed to conserve heat and reduce heating bill costs. Lack of fresh air circulation increases the viral density and risk of illness severity (Foster, 2014). See the superb graphic illustration by Bartzokas et al (Feb 26, 2021).in the New York Times of virus concentration in schools when the windows are opened. https://www.nytimes.com/interactive/2021/02/26/science/reopen-schools-safety-ventilation.html?smid=em-share
- Use an exhaust fans to ventilate a building. By continuously replacing the inside “stale” air with fresh outside air, the concentration of the virus in the air is reduced.
- Use High-efficiency particulate air (HEPA) air purifiers to filter the air within a room. These devices will filter out particles whose diameter is equal to 0.3 µ m. They will not totally filter out the virus; however, they will reduce it.
- Avoid buildings with recycled air unless the heating and air conditioning system (HAC) uses HEPA filters.
- Wear masks to protect other people and your community. The mask will reduce the shedding of the virus to others by people with COVID-19 or those who are asymptomatic carriers. This is superbly illustrated by Prather, Wang, & Schooley (2020) that not masking maximizes exposure, whereas universal masking results in the least exposure.

- Avoid long-term exposure to air pollution. People exposed to high levels of air pollution and fine particulate matter (PM2.5) are more at risk to develop chronic respiratory conditions and COVID-19 death rates. In the 2003 study of SARS, ecologic analysis conducted among 5 regions in China with 100 or more SARS cases showed that case fatality rate increased with the increment of air pollution index (Cui, Zhang, Froines, et al. , 2003). The higher the concentration of fine particulate matter (PM2.5), the higher the death rate (Conticini, Frediani, & Caro, 2020). As researchers, Xiao Wu, Rachel C. Nethery and colleagues (2020) from the Harvard T.H. Chan School of Public Health point out, “A small increase in long-term exposure to PM2.5 leads to a large increase in COVID-19 death rate, with the magnitude of increase 20 times that observed for PM2.5 and all cause mortality. The study results underscore the importance of continuing to enforce existing air pollution regulations to protect human health both during and after the COVID-19 crisis.“
- Breathe only through your nose. The nose filters, warms, moisturizes and slows the airflow so that airway irritation is reduced. Nasal breathing increases nitric oxide production that significantly increases oxygen absorption in the body. During inspiration through the nose the nitric oxide helps dilate the airways in your lungs and blood vessels (McKeown, 2016). More importantly for dealing with COVID-19, Nitric Oxide, produced and released inside the nasal cavities and the lining of the blood vessels, acts as an antiviral and a secondary strategy to protect against viral infections (Mehta, Ashkar & Mossman, 2012).
How to strengthen your immune system to fight the virus
The immune system is dynamic and many factors as well as individual differences affect its ability to fight the virus. It is possible that a 40 year-old person may have an immune systems that functions as a 70 year old, while some 70 year-olds have an immune system that function as a 40 year-old. Factors that contribute to immune competence include genetics, aging, previous virus exposure, and lifestyle (Lawton, 2020).
It is estimated that 70-80% mortality caused by Covid-19 occurred in people with comorbidity who are: over 65, male, lower socioeconomic status (SES), non white, overweight/obesity, cardiovascular heart disease, and immunocompromised. Although children comprised only a small percentage of the seriously ill patients, 83% of those children in the intensive care units had comorbidities and 60% were obese. The majority of contributing factors to comorbidities and obesity are the result of economic inequality and life style patterns such as the Western inflammatory diet (Shekerdemian et al, 2020; Zachariah, 2020; Pollan, 2020).
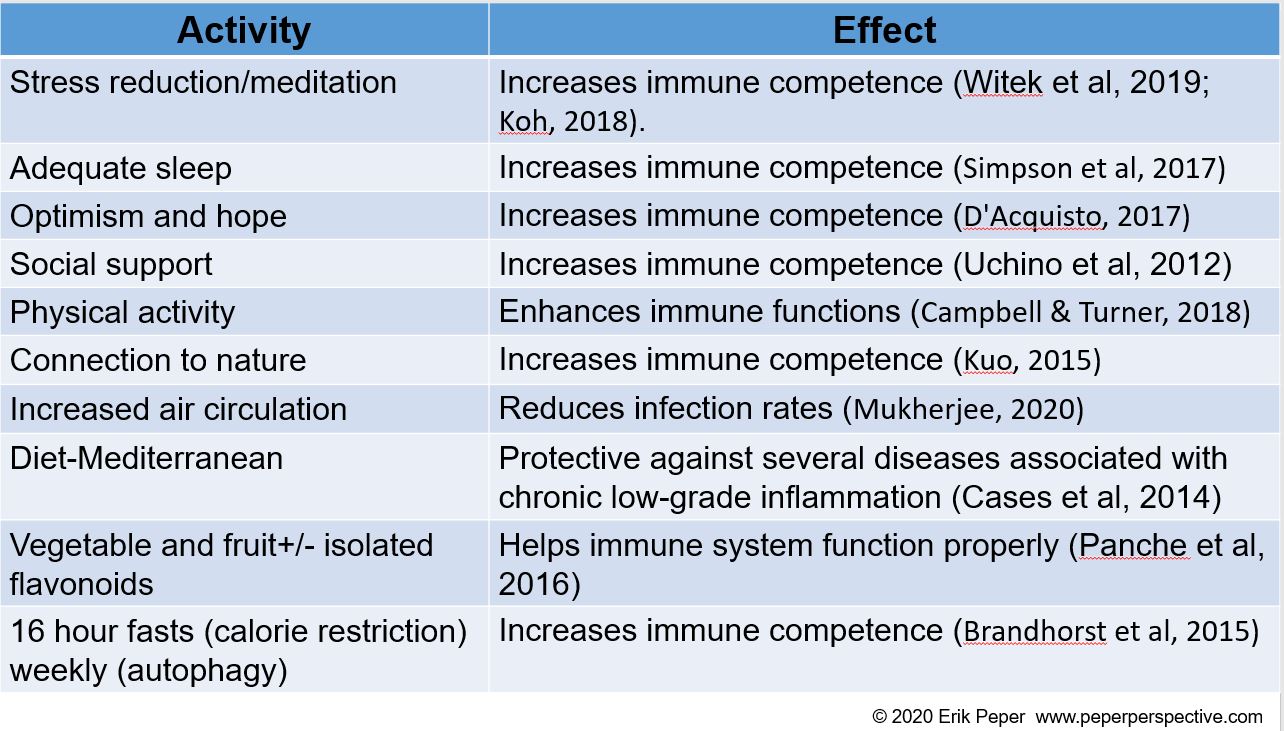
By taking charge of your lifestyle habits through an integrated approach, you may be able to strengthen your immune system (Alschuler et al, 2020; Lawton, 2020). The following tables, adapted from the published articles by Lawton (2020), Alschuler et al, (2020) and Jaffe (2020), list factors that support or decrease the immune system.
Factors that decrease immune competence

Factors that support immune competence
Phytochemicals and vitamins that support immune competence

REFERENCES
Abhanon, T. (2020 March 26). Practical tips how to keep yourself safe.
Foster, H. (2014 December 1). The reason for the season: why flu strikes in winter. SITN Science in the News.
Furukawa, N.W., Brooks, J.T., & Sobel, J. (2020, July). Evidence supporting transmission of severe acute respiratory syndrome coronavirus 2 while presymptomatic or asymptomatic. Emerg Infect Dis. [June3, 2020]. https://doi.org/10.3201/eid2607.201595
Ganyani, T., Kremer, C., Chen, D., Torneri, A, Faes, C., Wallinga, J., & Hensm N. (2020). Estimating the generation interval for COVID-19 based on symptom onset data doi:https://doi.org/10.1101/2020.03.05.20031815
Gaiha, S.M., Cheng, J. & Halpern-Felsher, B. (2020). Association between youth smoking, electronic cigarette use, and coronavirus disease 2019. Journal of Adolescent Health. Published online August 11, 2020. doi: https://doi.org/10.1016/j.jadohealth.2020.07.002
Heid, M. (2020-04-09). The Germ-Cleaning Power of an Open Window. Elemental by Medium.
BMJ Open, 11:e047474. http://dx.doi.org/10.1136/bmjopen-2020-047474
Jaffe, R. (2020). Reduce risk, boost immunity defense and repair abilities, and stay resilient. PERQUE Integrative Health.
Lawton, G. (2020). You’re only as young as your immune system. New Scientist, 245(3275), 44-48.
Lee, G. Y., & Han, S. N. (2018). The Role of Vitamin E in Immunity. Nutrients, 10(11), 1614.
Malaguarnera L. (2019). Influence of Resveratrol on the Immune Response. Nutrients, 11(5), 946.
Mukherjee, S. (2020). How does the coronavirus behave inside a patient? We’ve counted the viral spread across peoples; now we need to count it within people. The New Yorker, April 6, 2020.
Cell phone radio frequency radiation increases cancer risk*
Posted: November 12, 2018 Filed under: cancer, digital devices, self-healing, Uncategorized | Tags: cellphones, digital devices, Radio frequency radiation, technology 3 CommentsBe safe rather than sorry. Cellphone radio frequency radiation is harmful!
The National Toxicology Program (NTP) released on October 31, 2018 their final report on rat and mouse studies of radio frequency radiation like that used with cellphones. The $30 million NTP studies took more than 10 years to complete and are the most comprehensive assessments to date of health effects in animals exposed to Radio Frequency Radiation (RFR) with modulations used in 2G and 3G cell phones. 2G and 3G networks were standard when the studies were designed and are still used for phone calls and texting.
The report concluded there is clear evidence that male rats exposed to high levels of radio frequency radiation (RFR) like that used in 2G and 3G cell phones developed cancerous heart tumors, according to final reports. There was also some evidence of tumors in the brain and adrenal gland of exposed male rats. For female rats, and male and female mice, the evidence was equivocal as to whether cancers observed were associated with exposure to RFR.
“The exposures used in the studies cannot be compared directly to the exposure that humans experience when using a cell phone,” said John Bucher, Ph.D., NTP senior scientist. “In our studies, rats and mice received radio frequency radiation across their whole bodies. By contrast, people are mostly exposed in specific local tissues close to where they hold the phone. In addition, the exposure levels and durations in our studies were greater than what people experience.”
In the NTP study, the lowest exposure level used in the studies was equal to the maximum local tissue exposure currently allowed for cell phone users. This power level rarely occurs with typical cell phone use. The highest exposure level in the studies was four times higher than the maximum power level permitted. Butcher state, “We believe that the link between radio frequency radiation and tumors in male rats is real, and the external experts agreed.”
I interpret that their results support the previous–often contested–observations that brain cancers were more prevalent in high cell phone users especially on the side of the head they held the cellphone.
More some women who have habitually stashed their cell phone in their bra have been diagnosed with a rare breast cancer located beneath the area of the breast where they stored their cell phone. Watch the heart breaking TV interview with Tiffany. She was 21 years old when she developed breast cancer which was located right beneath the breast were she had kept her cell phone against her bare skin for the last 6 years.
While these rare cases could have occurred by chance, they could also be an early indicator of risk. Previously, most research studies were based upon older adults who have tended to use their mobile phone much less than most young people today. The average age a person acquires a mobile phone is ten years old (this data was from 2016 and many children now have cellphones even earlier). Often infants and toddlers are entertained by smartphones and tablets–the new technological babysitter. The possible risk may be much greater for a young people since their bodies and brains are still growing rapidly. I wonder if the antenna radiation may be one of the many initiators or promoters of later onset cancers. We will not know the answer; since, most cancer take twenty or more years to develop.
What can you do to reduce risk?
Act now and reduce the exposure to the antenna radiation by implementing the following suggestions:
- Keep your phone, tablet or laptop in your purse, backpack or briefcase. Do not keep it on or close to your body.
- Use the speakerphone or earphones with microphone while talking. Do not hold it against the side of your head, close to your breast or on your lap.
- Text while the phone is on a book or on a table away from your body.
- Put the tablet and laptop on a table and away from the genitals.
- Set the phone to airplane mode.
- Be old fashioned and use a cable to connect to your home router instead of relying on the WiFi connection.
- Keep your calls short and enjoy the people in person.
- Support legislation to label wireless devices with a legible statement of possible risk and the specific absorption rate (SAR) value. Generally, higher the SAR value, the higher the exposure to antenna radiation.
- Support the work by the Environmental Health Trust.
For an radio interview on this topic, listen to my interview on Deborah Quilter’s radio show. http://www.blogtalkradio.com/rsihelp/2018/11/20/why-you-should-keep-your-cell-phone-away-from-your-body-with-dr-erik-peper
For more information on NTP study see:
*The blog is adapted in part from the November 1, 2018 news release from the National Toxicology Program (NTP)1, National Institute of Environmental Health Sciences2, National Institute of Health (NIH)3.
- About the National Toxicology Program (NTP):NTP is a federal, interagency program headquartered at NIEHS, whose goal is to safeguard the public by identifying substances in the environment that may affect human health. For more information about NTP and its programs, visit niehs.nih.gov.
- About the National Institute of Environmental Health Sciences (NIEHS): NIEHS supports research to understand the effects of the environment on human health and is part of NIH. For more information on environmental health topics, visit niehs.nih.gov. Subscribe to one or more of the NIEHS news lists (www.niehs.nih.gov/news/newsroom/newslist/index.cfm) to stay current on NIEHS news, press releases, grant opportunities, training, events, and publications.
- About the National Institutes of Health (NIH):NIH, the nation’s medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit nih.gov.
Surgery: Hope for the best and plan for the worst!
Posted: March 18, 2018 Filed under: Pain/discomfort, placebo, self-healing, stress management, surgery, Uncategorized | Tags: anesthesia, hernia, iatrogenic illness, technology, urinary retention 20 CommentsAdapted from: Peper, E. Surviving and preventing medical errors. (2019). Townsend Letter-The Examiner of Alternative Medicine. 429, 63-69. https://townsendletter.com/surviving-and-preventing-medical-errors-peper/
The purpose of this blog is to share what I have learned from a cascade of medical errors that happen much more commonly than surgeons, hospitals, or health care providers acknowledge and is the third leading cause of death in the US (Makary, M.A. & Daniel, M., 2016). My goal here is to provide a few simple recommendations to reduce these errors.

It is now two years since my own surgery—double hernia repair by laparoscopy. The recovery predicted by my surgeon, “In a week you can go swimming again,” turned out to be totally incorrect.
Six weeks after the surgery, I was still lugging a Foley catheter with a leg collection bag that drained my bladder. I had swelling due to blood clots in the abdominal area around my belly button, severe abdominal cramping, and at times, overwhelming spasms. For six weeks my throat was hoarse following the intubation. Instead of swimming, hiking, walking, working, and making love with my wife, I was totally incapacitated, unable to work, travel, or exercise. I had to lie down every few hours to reduce the pain and the spasms.
Instead of going to Japan for a research project, I had to cancel my trip. Rather than teaching my class at the University, I had another faculty member teach for me. I am a fairly athletic guy—I swim several times a week, bike the Berkeley hills, and hiked. Yet after the surgery, I avoided even walking in order to minimize the pain. I moved about as if I were crippled. Now two years later, I finally feel healthy again.
How come my experiences were not what the surgeon promised?
All those who cared for me during this journey were compassionate individuals, committed to doing their best, including the emergency staff, the nurses, my two primary physicians, my surgeon, and my urologist. However, given the personal, professional, and economic cost to me and my family, I feel it is important to assess where things went wrong. The research literature makes it clear that my experience was by no means unique, so I have summarized some of the most important factors that contributed to these unexpected complications, following “simple arthroscopic surgery.”
- Underestimating the risk. Although the surgeon suggested that the operation would be very low risk with no complications, the published research data does not support his optimistic statement and misrepresented the actual risk. Complications for laparoscopic surgery range from 15% to as high as 38% or higher, depending on the age of the patient and how well they do with general anesthesia (Vigneswaran et al, 2015; Neumayer et al, 2004; Perugini & Callery, 2001). Experienced surgeons who have done more than 250 laparoscopic surgeries have a lower complication rate. However, a 2011 Cochran review points out that there is theoretically a higher risk that intra-abdominal organs will be injured during a laparoscopic procedure (Sauerland, 2011). In addition, bilateral laparoscopic hernia repair has significantly higher risk than single sided laparoscopic hernia repair for post-operative urinary retention (Blair et al, 2016). My experience is not an outlier–it is more common.
- Inappropriate post-operative procedures. In my case I was released directly after waking up from general anesthesia without checking to determine whether I could urinate or not. The medical staff and facility should never have released me, since older males have a 30% or higher probability that urinary retention will occur after general anesthesia. However, it was a Friday afternoon and the staff probably wanted to go home since the facility closes at 5:30 pm. This landed me in the Emergency Room.
- Medical negligence. In my case the surgeon recommended that I have my bladder in the emergency room emptied and then go home. That was not sufficient, and my body still was not working properly, requiring a second visit to the ER and the insertion of a Foley catheter. Following the second ER visit, the surgeon removed the catheter in his office in the late afternoon and did not check to determine whether I could urinate or not. This resulted in a third ER visit.
- Medical error. On my third visit to the emergency room, the nurse made the error of inflating the Foley catheter balloon when it was in the urethra (rather than the bladder) which caused tearing and bleeding of the urethra and possible irritation to the prostate.
- Drawbacks of the ER as the primary resource for post-surgical care. Care is not scheduled for the patient’s needs, but rather based on a triage system. In my case I had to wait sometimes two hours or more until a catheter could be inserted. The wait kept increasing the urine volume which expanded and irritated the bladder further.
- A medical system that does not track treatment outcomes. Without good follow-up and long-term data, no one is accountable or responsible.
- A reimbursement system that rewards lower up-front costs. The system favors quick outpatient surgeries without factoring in the long-term costs and harm of the type I experienced.
Assuming the best and not planning for the worst.
Can I trust the health care provider’s statement that the procedure is low risk and that the recovery will go smoothly?
The typical outcome of a medical procedure or surgery may be significantly worse than generally reported by hospitals or medical staff. In many cases there is no systematic follow-up nor data on outcomes and complications, thus no one knows the actual risks.
In the United States medical error results in at least 98,000 unnecessary deaths each year and 1,000,000 excess injuries (Weingart et al, 2000; Khon et al, 2000). The Institute of Medicine reported in 2012 that one-third of hospitalized patients are harmed during their stay (Ferguson, 2012; Institute of Medicine, 2012).
One should also be intelligently skeptical about positive claims for any specific study—it is important to know whether the study has been replicated with other populations and not just a particular group of patients.
To quote Dr. Marcia Angell (2009), the first woman editor of the highly respected New England Journal of Medicine, “It is simply no longer possible to believe much of the clinical research that is published, or to rely on the judgment of trusted physicians or authoritative medical guidelines. I take no pleasure in this conclusion, which I reached slowly and reluctantly over my two decades as an editor of The New England Journal of Medicine.”
The evidence for many procedures and medications is surprisingly limited
- Research studies frequently select specific subsets of patients. They may exclude many patients who have other co-morbidities.
- Clinical trials may demonstrate statistical significance without providing clinically meaningful results. For example, between 2009 and 2013 all most all cancer drugs that were approved for treatment in Europe showed upon follow-up no clear evidence that they improved survival or quality of life for patients (Davis et al, 2017; Kim & Prasad, 2015).
- Pharmaceuticals are tested only against a passive placebo. In some cases, the patient’s positive response may actually be the placebo effect, due to physical sensations induced by the medication or its side effects, thus inspiring hope that the drug is working (Peper and Harvey, 2017).
- Negative side effects are significantly underreported. The data depend on self-report by both the patient and the health care provider.
Many published studies on the positive clinical outcome of pharmaceuticals are suspect. As Dr. Richard Horton (2015), Editor-in-Chief of The Lancet, wrote in 2015, “A lot of what is published is incorrect … much of the scientific literature, perhaps half, may simply be untrue. Afflicted by studies with small sample sizes, tiny effects, invalid exploratory analyses, and flagrant conflicts of interest, together with an obsession for pursuing fashionable trends of dubious importance, science has taken a turn towards darkness.”
Most studies, including those on surgery, lack long-term follow-up.
The apparent short-term benefits may be not beneficial in the long term or may even be harmful. For example, doctors and patients are convinced that SSRIs (serotonin re-uptake inhibitors—antidepressants such as Paxil and Prozac) are beneficial, with resulting global sales in 2011 of $11.9 billion. However, when all the research data were pooled, metanalysis showed that these drugs are no more effective than placebo for the treatment of mild to moderate depression and increase suicides significantly among young adults (Fournier et all, 2010; Kirsch, 2014).
Consider long-term follow-up in my case: the surgeon will report a successful surgery, despite the fact that it took me almost two years to recover fully. (I did not die during surgery and left in seemingly good shape.). Although I called him numerous times for medical guidance during my complications, the outpatient surgical facility will report no complications since I was not transferred from that facility during the surgery to a hospital for continuing care. My insurance carrier that paid the majority of the medical bills recorded the invoices as separate unrelated events: one surgery/one bill, but three separate bills for the emergency room, an additional visit to my primary care physician to check my abdomen when my surgeon did not return my call, and the ongoing invoices from the urologist. They all reported success because the iatrogenic events were not linked to the initial procedure in the data base.
In my case, following surgery, I had to go to the emergency room on three separate occasions due to post-operative urinary retention, placing me at risk of permanent detrusor muscle damage. For more than 18 months, I was under the care of a urologist.
Over the past two years, my symptoms have included gastrointestinal inflammation, spasms, and abdominal bulging, which are only now disappearing. Even my posture has changed. I am now working to reverse the automatic flexing at the hips and leaning forward which I covertly learned to reduce the abdominal discomfort. This level of discomfort and dysfunction are new to me. Reading the research on laparoscopy, I realized that excessive internal bruising, large hematomas, and internal adhesions are fairly common with this type of surgery. However, soft tissue injuries are difficult to confirm with imaging techniques.
My complications were also a direct result of inappropriate post-surgical recommendations and treatment. The symptoms were further compounded by faulty patient discharge procedures performed by the outpatient surgical facility. Since this was my first general anesthesia, I had no idea that I would be one of the people whose outcome were not what the surgeon had predicted. Thus, hope for the best, but plan for the worst.
SCHEDULING MEDICAL PROCEDURES
The following are recommendations may help reduce post-surgical or medical procedure complications.
- Schedule elective medical procedures or surgery early the morning and in the middle of the week. Do not schedule procedures on Mondays, Fridays, or in the afternoon. Procedures performed in the afternoon have significant increase in complications and errors. Anesthesia complications, for example, are four times higher in the afternoon than in the morning (Wright et al, 2006). Our biological rhythms affect our ability to attend and focus. In the morning most people are able to concentrate better than in the afternoon (Pink, 2018).
- Avoid weekends. Procedures performed on weekends (as compared to those done in the middle of the week) increase the risk of complications or dying. For example, babies born on the weekend have a 9.2% higher infant mortality than those born during the week, while those born on Tuesdays have the lowest death rate (Palmer et al, 2015). It is possible that on Mondays medical staff are recovering from weekend binging, while on Fridays they are tired and looking forward to the weekend? If elective procedures are done on a Friday and complications arise, the emergency room is the only option, as the medical staff may not be available over the weekend. In my case the procedure was done on a Friday, and I left the surgical outpatient facility at 2 pm. When complications occurred, it was after 5:30 pm—phone support from the advice nurse and the surgeon on call were my only option until the following Monday. Thus, I had to go the emergency room late Friday evening and again the next evening because of urinary retention, with a long delay in a busy waiting room. Since, I wasn’t bleeding or having a heart attack, that meant I had to wait, wait and wait, which significantly aggravated my specific problem.
- Schedule medical procedures at least one or two weeks before any holiday. Do not schedule surgery just before or during holidays. Medical staff also take holidays and may not available. In my case, I scheduled the procedure the Friday before Thanksgiving because I thought I would have a week of recovery during my Thanksgiving break from teaching. This meant that medical staff were less available and more involved in their holiday planning.
- Schedule procedures so that you are released early in the day. This can allow you to return to the facility in case complications arise. I was released at 2 pm and the complications did not occur until early evening. The facility was closed, so the only option was the ER. When possible, schedule medical procedures or surgery in a facility that is able to provide post-operative care after 5 pm.
- Do not schedule elective procedures during the month of July in an academic teaching hospital. During this month mortality increases and efficiency of care decreases because of the end of the academic year and subsequent changeover to new personnel (Young et al, 2011). Medical school graduates with limited clinical experience begin their residencies and experienced house staff are replaced with new trainees. This is known as the July effect in the U.S. and Killing season in the United Kingdom. During the month of July in any given year, fatal medication errors, for example, increase by 10% at teaching hospitals, but not at neighboring hospitals which do not experience this turnover in medical personnel (Phillips & Barker, 2010).
- Have procedures performed at a medical facility in which the health care professional has no financial interest—take economics out of the equation. When health care practitioners have financial interest in a facility, they tend to order more tests and procedures than health care providers who have no financial interests (Bishop et al, 2010). In my case the surgeon had a financial interest in the outpatient surgical facility where I received surgery. Had I had the operation across the street in the hospital where the surgeon also operates, I probably would not have been released early, avoiding the problems in follow-up care.
STRATEGIES TO OPTIMIZE OUTCOMES AND HEALTH
Organize your support system. Assume that recovery could be more difficult then promised.
Before your procedure, ask family members, friends, and neighbors to be prepared to help. If you did not need them, thank them for their willingness to help. In my case I did not plan for complications, thus my wife was my entire support system, especially for the first three weeks when I was unable to do anything except rest and cope. I was very fortunate to have numerous family, friends, and colleagues who offered their expertise to help me understand what was going on and who assumed my responsibilities when needed.[1]
- Bring an advocate to your appointments. Have your advocate/friend keep notes and ask questions, especially if the health care provider is a respected authority and you are suffering, exhausted, and/or anxious. Record any detailed instructions you must follow at home as a video or audio file on your cell phone or write them down (be sure to ask the health provider for permission). Under stress one may not be able to fully process instructions from the health care provider.
- Make a list of questions and concerns before seeing your health care provider. Talk to your partner and close friends and ask them if there are questions or concerns that you should raise with your provider.
- Ask for more information when tests or procedures are proposed (Robin, 1984).
- Why do you recommend this particular test/procedure/intervention for me and what are the major benefits?
- What are the risks and how often do they occur, in your experience and in the research literature?
- What will you do if the treatment is not successful?
- Ask your provider if there is anything that you should or should not do to promote healing. As much as possible, ask for advice on specific efforts you can make. General statements without instructions such as, “Relax” or “Don’t worry,” are not helpful unless the practitioner teaches you specific skills to relax or to interrupt worrisome thoughts. Many health professionals do not have the time to teach you these types of skills. In many cases the provider may not be able to recommend documented peer-reviewed self-care strategies. Often they imply—and they can be correct—that the specific medical treatment is the only thing that will make you better. In my case I did not find any alternative procedures that would reverse a hernia, although there may be habitual postural and movement patterns that could possibly prevent the occurrence of a hernia (Bowman, 2016). Being totally dependent upon the medical procedure may leave you feeling powerless, helpless, and prone to worry. In most cases there are things you can do to optimize self- healing.
- Think outside the box. Explore other forms of self-care that could enhance your healing. Initiate self-care action instead of waiting passively. By taking the initiative, you gain a sense of control, which tends to enhance your immune system and healing potential. Do anything that may be helpful, as long as it is not harmful. In my case, future medical options to resolve urinary retention could include additional medications or even surgery. Researching the medical literature, there were a number of studies showing that certain herbs in traditional Chinese medicine and Ayurveda medicine could help to reduce prostate inflammation and possibly promote healing. Thus, I began taking three different herbal substances for which there was documented scientific literature. I also was prescribed herbal tea to sooth the bladder. Additionally, I reduced my sugar and caffeine intake to lower the risk of bladder irritation and infection.
- Collaborate with your health care provider. Let your provider know the other approaches you are using. Report any interventions such as vitamins, herbs, Chinese medicine. Ask if they know of any harm that could occur. In most cases there is no harm. The health care professional may just think it is a waste of time and money. However, if you find it helpful, if it gives you control, if it makes you less anxious, and if it is not harmful, it may be beneficial. What do you have to lose?
- Assume that all the health care professionals are committed to improving your health to the best of their ability. Yet at times professionals are now so specialized that they focus only on their own discipline and not the whole person. In their quest to treat the specific problem, they may lose sight of other important aspects of care. Thus, hope for the best, but plan for the worst.
PREPARING FOR SURGERY
Assume that the clinical staff will predict a more positive outcome than that reported in the medical literature. In most cases, especially in the United States, there is no systematic follow-up data since many post-surgical complications are resolved at another location. In addition, many studies are funded by medical companies which have a vested interest and report only the positive outcomes. The companies tend not to investigate for negative side-affects, especially if the iatrogenic effects occur weeks, months, or years after the procedure. This has also been observed in the pharmaceutical companies sponsoring studies for new medications.
Generally, when independent researchers investigated medical procedures they found the complication rate three-fold higher than the medical staff reported. For example, for endoscopic procedures such colonoscopies, doctors reported only 31 complications from 6,383 outpatient upper endoscopies and 11,632 outpatient colonoscopies. The actual rate was 134 trips to the emergency room and 76 hospitalizations. This discrepancy occurred because the only incidents reported involved patients who went back to their own doctors. It did not capture those patients who sought help at other locations or hospitals (Leffler et al, 2010).
The data are even worse for patients who are hospitalized; in the U.S. 20% of patients who leave the hospital return within a month while in England, 7% of those leaving the hospital return within a month (Krumholz, 2013).
- Ask about possible complications that could arise, the symptoms, and what the physician would do if they occurred. Do not assume the health professional will have the time to explain or know all the possible complications. In my case when the surgeon removed the catheter at 4 pm during my second emergency room visit, I had to ask, “What would happen if I still cannot urinate?” Again, the emergency room was the only answer. However, I know now that he could have taught me simple self-catherization which would have eliminated the long waiting in the emergency room, the excessive stretching of the bladder and the subsequent emergency room medical error on my third visit to the ER. It would also have reduced the medical costs by a thousand-fold.
- Get a second opinion. In my case, the surgeon came highly recommended, is very experienced, and has done many hernia repairs. I trusted his judgement that I needed a bilateral hernia repair although I only felt the bulging in the right inguinal area and did not feel bulging or sensations in the left inguinal area. Despite my feeling of trust, I should have asked for a second independent opinion just to be sure. In many moments of despair when suffering the significant complications, I even started to wonder if the bilateral laparoscopic surgical repair was really necessary or just done to increase the income of the surgeon and the outpatient surgical facility in which he had a financial interest. My surgery resulted in large hematomas, irritation of internal organs, and possible damage to the GI track. This type of complication did not occur for a close friend who had a single-sided hernia repair by the same surgeon in a hospital where the surgeon had no financial interests.
- Request medical personnel who are highly experienced in the intervention. Mortality and complications rates are significantly lower for practitioners who have done the procedure at least 250 times.
- Don’t assume the worst but be prepared for the worst. Ask your health care provider about the various side effects of surgery, including the worst things that could happen, and then develop a pre-emptive plan.
The most common problems associated with surgery and general anesthesia include:
- Urinary retention. Following general anesthesia, neural enervation to the bladder and gastrointestinal tract are often affected. The general risk for postoperative urinary retention (POUR) for all types of surgeries ranges from 7% to 52% (Tammela et al, 1986; Petros et al, 1990; Petros et al, 1991; Gonullu et al, 1993; Tammela, 1995). For patients who have surgery for hernia repair 24.4% will experience postoperative urinary retention (Keita et al, 2005)—one in four. The risk for older males is even higher (Blair et al, 2017). Do not leave the medical unit until you have urinated or have a Foley catheter inserted with a leg bag and appropriate follow-up managed by a urologist. In my case, neither the surgeon nor the outpatient hospital checked to determine whether I could urinate—they just discharged me the moment I was conscious. Discharging a patient who has had general anesthesia without checking to determine whether they can urinate goes against all medical guidelines and standard hospital policies and constitutes malpractice. As this was my first surgery, I had no idea that urinary retention could occur. Thus, I did not recognize the symptoms nor did the advice nurse or the surgeon when called for advice before I checked into the emergency room.
- Expect constipation and plan to eat a high roughage diet that supports bowel movements. In case bowel function is slow in resuming, you may want to have on hand simple over-the-counter supplements such as magnesium capsules, psyllium husks, and aloe vera juice or gel, all available at any health food store. Liquid magnesium citrate (GoLytely® solution available at drug stores), can be useful, but tends to be a little stressful to take. Check these over-the-counter supplements with your provider to avoid supplement-drug interaction.
- Infection. Many patients pickup hospital-induced infections (nosocomial infections). In my case, I after four weeks with a Foley catheter, I got a mild bladder infection and had to control it with antibiotics. While in the hospital, avoid direct physical contact with other patients and staff, wash and rewash your hands. Remember medical staff tend are less attentive and wash their hands 10% less in the afternoons than in morning. Ask the medical staff to thoroughly wash their hands before they examine you. If you do get an infection, contact your medical provider immediately.
ACTION STEPS
- Pace yourself. Assume that recovery could be slower than promised. Although your body may appear to be healed, in many cases your vitality could be significantly reduced for a number of months, and you will probably feel much more fatigued in the evening. The recovery from general anesthesia has been compared to recovery from a head-on car collision.
- Identify your support system in case you cannot take care of yourself initially. Organize family and friends to help you. In my case, for the first two weeks I did not have the energy to do anything for myself—the overwhelming abdominal spasms and the three episodes in the ER had drained my energy. I was very lucky that I had my family and friends to help me. For the first few weeks I was so distracted by the pain and discomfort that I did not drive or take care of myself.
- Have a plan in case you need to go to the Emergency Room in the evening. Know its location and have someone who can take you.
- Assume that you will probably have an extensive wait in the ER unless you are desperately ill. Do not try to “tough it out.” Be totally honest about your level of pain, so you can get the best possible care. In my case, I had horrible abdominal pain and spasms with urinary retention, but still acted as if I were okay. When the admitting nurse asked me how I felt, I rated my discomfort as a 5 on a scale from 0 to 10. In my mind I compared the pain with that I had experienced after a skiing accident, which was much worse. What I had forgotten was that the ER is triage system, so I had to wait and wait and wait, which was phenomenally uncomfortable.
- In the ER, ask which medical specialist can follow up with you if further issues develop. A general hospital usually has specialists on call. In my case, if I had requested care from a specialist, I would have been treated directly by a urologist. I would not have had to follow the advice of the surgeon who said, “When you go to Emergency Room, have them empty the bladder and then go home.” Almost all urologists would have recommended keeping the Foley catheter in for a few days to allow the side effects of the anesthesia and the trauma caused by the bladder expansion to ameliorate and then test whether urination was possible.
- Have a medical advocate with you at all times who can observe that the procedures are done correctly. There is a four-fold increase in errors during the evenings and nights as compared to the morning. The more medical staff is multi-tasking, the more likely they will make errors. Have the medical personnel explain any procedure before they perform it—why and how they will do the procedure and what you will experience. You also need to know if they are experienced in that particular procedure? If the answers do not make sense, stop them and ask for another staff member.
- In the ER, record the instructions on your phone. Have medical staff explain and demonstrate to you and your support person what you will need to do at home. Then repeat the instructions back to them to be certain you have it right.
- Remind yourself that errors can occur. In my case, during the third ER visit for urinary retention, the nurse delayed the anchoring of the catheter and it had slipped down into the urethra. As she began to pump, I could feel my urethra tearing and I alerted her to stop. This was immediately followed by another procedural error on her part, so I had to again alert her to stop, which she finally did. All this occurred at 1 am in the morning. As the patient, I had to take charge at a time when I was totally exhausted. As the nurse retreated, I was left sitting on the gurney waiting for someone to come and follow-up. I waited and waited and when I finally stood up, the catheter dropped out and I began bleeding.
Lesson learned: hope for the best but prepare for the worst. In my situation, after eight weeks and numerous visits to the urologist, he removed the catheter. He did this at 8:30 in the morning. This way I could go home and in case something happened, I could go back to his office for further care. Before leaving the office, I planned for the worst. I asked what would happen if I could not urinate later in the evening and requested that he give me a few catheters, so if problems developed, I could catheterize myself.
The urologist gave me the catheters and explained how to use them, although I did not actually practice on myself. Still, I felt better prepared. During the day, I become more and more optimistic because I had no problems; however, at 2 am I woke up unable to urinate. For the next hour, I felt very anxious about inserting the catheter, since I had never done it myself. Finally, my discomfort overcame my anxiety. To my surprise, it was easy. After waiting a few minutes, I removed the catheter and went to bed feeling much more comfortable. The next morning after breakfast and a cup of coffee, I found that my body was working fine without the catheter.
Had I not planned for the worst, I would have once again gone to the Emergency Room and probably waited for hours, risking a repeat of tremendous discomfort and irritation. This simple planning reduced my medical cost more than a thousand-fold from $1700 for the emergency room to $2 for some single-use catheters.
References
Angell, M. (2009). Drug companies & doctors: A story of corruption. The New York Review of Books, 56(1), 8-12. http://www.nybooks.com/articles/2009/01/15/drug-companies-doctorsa-story-of-corruption/
Bishop, T.F., Federman, A.D., & Ross, J.S. (2010). Laboratory test ordering at physician offices with and without on-site laboratories, Journal of Gen Intern Med, 25(10)m 1057-1063. doi: 10.1007/s11606-010-1409-7
Blair, A.B., Dwarakanath, A., Mehta, A., Liang, H., Hui, X., Wyman, C., Ouanes, J.P.P., & Nguyen, H.T. (2017). Postoperative urinary retention after inguinal hernia repair: a single institution experience. Hernia, 21(6), 895-900.
Bowman, K. (2016). Diastasis Recti: The whole-body solution to abdominal weakness and separation. Propriometrics Press: Carlsborg, WA 98324
Davis, C., Naci, N., Gurpinar, E., Poplavska, E., Pinto, A., Aggarwal, A. (2017). Availability of evidence of benefits on overall survival and quality of life of cancer drugs approved by European Medicines Agency: retrospective cohort study of drug approvals 2009-13. BMJ, 2017; j4530 DOI: 10.1136/bmj.j4530
Ferguson, T. B. (2012). The Institute of Medicine Committee report “best care at lower cost: the path to continuously learning health care”. Circulation: Cardiovascular Quality and Outcomes, 5(6), e93-e94. http://jama.jamanetwork.com/article.aspx?articleid=185157
Fournier, J. C., DeRubeis, R. J., Hollon, S. D., Dimidjian, S., Amsterdam, J. D., Shelton, R. C., & Fawcett, J. (2010). Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA, 303(1), 47-53.
Gönüllü, N. N., Gönüllü, M., Utkan, N. Z., Dülger, M., Gökgöz, S., & Karsli, B. (1993). Postoperative retention of urine in general surgical patients. The European journal of surgery= Acta chirurgica, 159(3), 145-147.
Horton, R. (2015). Offline: What is medicine’s 5 sigma? The Lancet, 385(9976), 1380. http://www.thelancet.com/journals/lancet/article/PIIS0140-6736%2815%2960696-1/fulltext?rss%3Dyes
Institute of Medicine’s Infographic, accompanying their 2012 report, Best Care at Lower Cost, at http://www.iom.edu/Reports/2012/Best-Care-at-Lower-Cost-The-Path-to-Continuously-Learning-Health-Care-in-America/Infographic.aspx
Keita, H., Diouf, E., Tubach, F., Brouwer, T., Dahmani, S., Mantz, J., & Desmonts, J. M. (2005). Predictive factors of early postoperative urinary retention in the postanesthesia care unit. Anesthesia & Analgesia, 101(2), 592-596.
Kim, C. & Prasad, V. (2015). Cancer drugs approved on the basis of a surrogate end point and subsequent overall survival-An analysis of 5 Years of US Food and Drug Administration approvals. JAMA Intern Med,175(12):1992-1994. doi:10.1001/jamainternmed.2015.5868
Kirsch, I. (2014). The emperor’s new drugs: medication and placebo in the treatment of depression. In Placebo (pp. 291-303). Springer Berlin Heidelberg.
Kohn, L. T., Corrigan, J. M., & Donaldson, M. S. (Eds.). (2000). To err is human: building a safer health system (Vol. 6). National Academies Press. http://www.ncbi.nlm.nih.gov/books/NBK221671/
Krumholz, H. M. (2013). Post-hospital syndrome—an acquired, transient condition of generalized risk. New England Journal of Medicine, 368(2), 100-102. http://www.nejm.org/doi/full/10.1056/NEJMp1212324
Leffler, D.A, Kheraj, R., Garud, S., Neeman, N., Nathanson, L.A., Kelly, C.P., Sawhney, M., Landon, B., Doyle, R., Rosenberg, S., & Aronson, M. (2010). The incidence and cost of unexpected hospital use after scheduled outpatient endoscopy. Arch Intern Medicine, 170(19), 1752-1757. http://archinte.jamanetwork.com/article.aspx?articleid=226125
Makary, M.A. & Daniel, M. (2016). Medical error–the third leading cause of death in the US. British Medical Journal, 353:i2139
Neumayer, L., Giobbie-Hurder, A., Jonasson, O., Fitzgibbons Jr, R., Dunlop, D., Gibbs, J., & Henderson, W. (2004). Open mesh versus laparoscopic mesh repair of inguinal hernia. New England Journal of Medicine, 350(18), 1819-1827. http://academicdepartments.musc.edu/surgery/education/resident_info/journal_club/09-10/April-inguinal.pdf
Palmer, W. L., Bottle, A., & Aylin, P. (2015). Association between day of delivery and obstetric outcomes: observational study. BMJ, 351, h5774. http://www.bmj.com/content/bmj/351/bmj.h5774.full.pdf
Peper, E. & Harvey, R. (2017). The fallacy of the placebo controlled clinical trials: Are positive outcomes the result indirect treatment side effects? NeuroRegulation. 4(3–4):102–113 2017 doi:10.15540/nr.4.3-4.102
Perugini, R. A., & Callery, M. P. (2001). Complications of laparoscopic surgery. http://www.ncbi.nlm.nih.gov/books/NBK6923/?report=reader
Petros, J. G., & Bradley, T. M. (1990). Factors influencing postoperative urinary retention in patients undergoing surgery for benign anorectal disease. The American Journal of Surgery, 159(4), 374-376.
Petros, J. G., Rimm, E. B., Robillard, R. J., & Argy, O. (1991). Factors influencing postoperative urinary retention in patients undergoing elective inguinal herniorrhaphy. The American Journal of Surgery, 161(4), 431-433.
Phillips, D. P., & Barker, G. E. (2010). A July spike in fatal medication errors: a possible effect of new medical residents. Journal of General Internal Medicine, 25(8), 774-779. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2896592/
Pink, D.H. (2018). When: The Scientific Secrets of Perfect Timing. New York: Riverhead Books, ISBN-13: 978-0735210622
Robins, E.D. (1984). Matter of Life & Death: Risks vs. Benefits of Medical Care. New York: W.H. Freeman and Company.
Sauerland, S., Walgenbach, M., Habermalz, B., Seiler, C. M., & Miserez, M. (2011). Laparoscopic versus open surgical techniques for ventral or incisional hernia repair. The Cochrane Library.
Tammela, T. (1994). Postoperative urinary retention–why the patient cannot void. Scandinavian Journal of Urology and Nephrology. Supplementum, 175, 75-77.
Tammela, T., Kontturi, M., & Lukkarinen, O. (1986). Postoperative urinary retention: I. Incidence and predisposing factors. Scandinavian Journal of Urology and Nephrology. 20(3), 197-201.
Vigneswaran, Y., Gitelis, M., Lapin, B., Denham, W., Linn, J., Carbray, J., & Ujiki, M. (2015). Elderly and octogenarian cohort: Comparable outcomes with nonelderly cohort after open or laparoscopic inguinal hernia repairs. Surgery, 158(4), 1137-1144.
Weingart, S. N., Wilson, R. M., Gibberd, R. W., & Harrison, B. (2000). Epidemiology of medical error. BMJ: British Medical Journal, 320(7237), 774. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1117772/
Wright, M.D., Philips-Bute, B., Mark, J.B., Stafford-Smith, M., Grichnik, K.P., Andregg, B.C., & Taekman, J.M. (2006). Time of day effects on the incidence of anesthetic adverse events. Quality and Safety in Health Care. 15(4): 258–263.doi: 10.1136/qshc.2005.017566
Young, J. Q., Ranji, S. R., Wachter, R. M., Lee, C. M., Niehaus, B., & Auerbach, A. D. (2011). “July effect”: impact of the academic year-end changeover on patient outcomes: a systematic review. Annals of Internal Medicine, 155(5), 309-315. http://www.girardslaw.com/library/July_Effect_Annals_of_Internal_Medicine.pdf
[1] I think my family, friends and colleagues (Karen Peper, Norihiro Muramatsu, Richard Harvey, David Wise, Annette Booiman, Lance Nagel and many others) who generously supported me during this journey.
Millennials and the impact of technology
Posted: November 29, 2016 Filed under: Uncategorized | Tags: cellphones, depression, health, millennials, productivity, technology 1 CommentTechnology connects us 24/7. Like a drug it provides instantaneous reinforcement when searching for information and sending or receiving social messages. Millennials are the first generation of digital natives who are always connected–from being jarred awake by their cellphone alarm to checking email or Facebook just before sleep. They are unlike their parents who are digital immigrants and have experienced face-to-face communication instead of virtual/digital communication. The video below, Simon Sinek on Millennials in the Workplace, offers an interesting insight of in the lives of millennials.



